
The HTI virtual research platform is made up of a curated selection of partner organisations. Innovators seeking support at any stage of translational hearing development can access this expertise and infrastructure by joining the HTI Network, and submitting an enquiry.
Our partner organisations
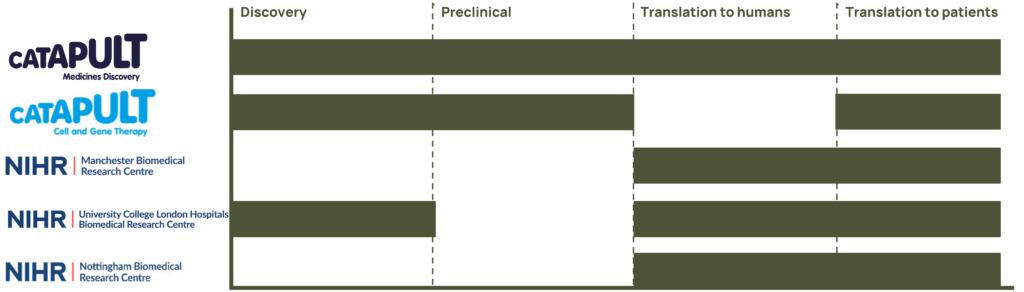
Medicines Discovery Catapult

Medicines Discovery Catapult is a national facility with the purpose to transform great UK science into better treatments through partnership. Established by Innovate UK, an agency of the UK government, Medicines Discovery Catapult is an independent not-for-profit organisation.
It brings together a fragmented sector of industry, academia, charities, technologists, services, finance companies, SMEs and start-ups, reshaping drug discovery through collaboration for patient benefit.
Medicines Discovery Catapult provides unique scientific capabilities and know-how in drug-discovery, facilitating access to specialist facilities, technology and expertise within the UK, supporting academic innovators and SMEs to deliver growth for the drug discovery economy, tackling industry-led challenges that limit today’s discovery processes and industrialising and driving adoption of new tools and techniques for discovering medicines.
Cell and Gene Therapy (CGT) Catapult

The Cell and Gene Therapy (CGT) Catapult is a centre of excellence in innovation, with the core purpose of building a world-leading cell and gene therapy sector in the UK as a key part of a global industry. Supported by Innovate UK, the CGT Catapult’s mission is to drive the growth of the industry by helping cell and gene therapy organisations across the world translate early stage research into commercially viable and investable therapies.
Based on the 12th floor of Guy’s Hospital in central London, with over 170 cell and gene therapy experts, state-of-the art development and viral vector laboratories. As well as a recently built £55m large-scale GMP manufacturing centre in Stevenage to help bring cell and gene therapies to market in the UK and internationally.
Manchester Biomedical Research Centre

Manchester BRC Hearing Health Theme is a world leading infrastructure for hearing research. Driving hearing health improvements through life-changing research that bridges the gap between new discoveries and individualised care. With the UK’s only Hearing Device Research Centre (HDRC), it drives innovation and accelerates the translation of technologies into the NHS.
The BRC partnership between the University of Manchester and Manchester University NHS Foundation Trust collaborates with national and international researchers, professional bodies and charities, to offer industry access to world class facilities. The trans-disciplinary team has an established portfolio that includes new discoveries in prevention, minimising risk, diagnosis and treatment.
Nottingham Biomedical Research Centre

Supported by NIHR infrastructure funding, the partnership between the University of Nottingham and Nottingham University Hospitals NHS Trust has since established itself as a flagship research centre addressing major clinical issues in the ENT and audiological management of hearing loss and tinnitus.
The Nottingham BRC Hearing Theme offers some of the best infrastructure in the UK for supporting early-phase translational research in the hearing sciences. In particular, the commitment is to pursue research through multi-disciplinary collaboration that can be translated into practical benefits for patients.
NIHR University College London Hospitals Biomedical Research Centre

The NIHR University College London Hospitals (UCLH) BRC Hearing Health theme builds on the unique partnership of the University College London (UCL) Ear Institute and the Royal National ENT and Eastman Dental Hospitals. Embedded in a world-leading research institute in one of the top ten universities in the world and Europe’s largest dedicated ENT hospital with close links to Great Ormond Street Children’s Hospital, the UCLH BRC provides a unique infrastructure and excellence for rapid translation of hearing discoveries into patient populations..
Leading some of the world’s first early phase trials of hearing therapeutics developed to protect and restore hearing, the UCLH BRC advises global biotechnology and pharmaceutical companies on the design and delivery of such trials. The BRC capability spans wet lab molecular and cellular research, translational auditory neuroscience, developing proof-of-concept to clinical trials, analysis of routine hearing health data, health economics and policy, thus supporting forward and reverse translation to advance the field of novel hearing therapeutics.
Become a partner
Does your organisation offer complementary infrastructure, capability and expertise at any stage of the hearing therapeutics pipeline?
Please get in touch by e-mailing us at hti@developer.rnid.org.uk